Isokinetic movement has been available since the late 1950’s. First introduced by Hettinger isokinetic movement was is in contrast to the other forms of motion in that it permitted maximum muscle contraction throughout the full range of joint motion.
Origionally referred to as ‘accommodating resistance exercise’. This new resistance was variable in proportion to the change in muscular capability at every point in the range of motion. The variation is controlled so that at all times it equals the product of the muscular strength.
The concept was far from an immediate a success. Isokinetics machines were few and far between with little or no feedback. The results we all love to hate were not available with exercise as the only option.
In 1967 J Perrine introduced a new speed controlled device which was described as ‘cybernetic exercise’. His first machine was called the Cybex 1. To see the original patent please press here (pdf format).
Lumex, Inc., a therapeutic health and hospital products company (founded in 1947), bought the patent for the first isokinetics machine in 1969 from Perrine. They set up the Cybex branch of the company and offered machines under various names (some peripheral joint and some combination motions). The equipment provided isokinetic resistance which accurately accommodated for the variable exertion applied by a muscle/s as the joint/s progressed through the full range of motion. Because isokinetic resistance gave constant load at pre set speed it had the benefit of not overloading the joint or musculo-skeletal unit through a full range of motion. The combination of resistance and speed control became a hit as it provided effective results in rehabilitation and exercise.
Lumex sold the Cybex (1) dynamometer
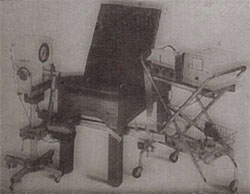
They also sold other physical rehabilitation and exercise products (isokinetic based, including exercise bikes etc.), such as the Fitron, and the Kinitron.

The Cybex Orthotron assessed ankle, knee, hip, and shoulder movements.

In 1974 Lumex created the Cybex division (later becoming the Cybex brand) the following year.
Isokinetic testing was originally a tool used mainly in exercise science and the only isokinetic movements available were concentric (with no thought given to isotonics, isometrics, continuous passive motion (CPM) or range of motion expansion). Eccentric actions were not attainable due to the nature of the dynamometer (the earliest dynamometers used oil passing through a variable sized hole to control resistance this gave smooth concentric resistance but could not be reversed to offer eccentric resistance)
Eventually, isokinetic testing found its way into therapy albeit with very rigid machines usually specific to a joint or small numbers of joints.
At the end of the 1970’s and into the early 1980’s there were major developments in the isokinetics world.
1980/1 saw the introduction of a printer (thermoprinter) to the new Cybex II this gave real time data for analysis and interpretation. Isokinetics machines had become interactive.
Computer Sports Medicine International ( CSMI ) president and CEO Rich Potash teamed with Cybex to design a MAC/PC based computer system for the new Cybex II
In 1982 Cybex saw international sales in over 20 countries. CSMI released the HUMAC System for the Cybex II
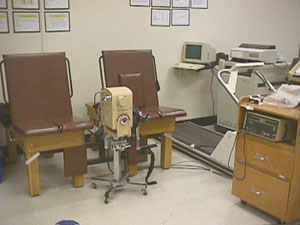
Based on the Apple //e the HUMAC was the first computer/software system to replace the Cybex II Chart Recorder. HUMAC was the first computerized isokinetic data analysis system for the MAC computer system. 1983 CSMI releases the HUMAC Computer System for the IBM PC integrating into the Cybex II main systems.
1982 Chattecx (Chattanooga TN) releases the Kin-Com (Kinematic Communicator) 500H. The 500H offered a revolution in isokinetics as it offered eccentric actions and was based on a two chair system.
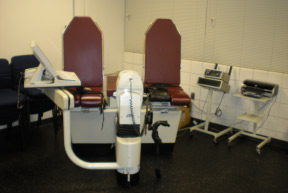
1985 Lido (Loredan Biomedical, Davis, CA) releases the Lido MJ (multi Joint) and Lido Linea (leg press) units in direct competition to the Cybex II.
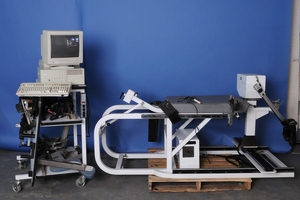
1986 CSMI releases HUMAC upgrades for the Cybex Orthotron II, KT-1, and KT-2 machines. The upgrades include custom torque and position sensors along with a modified version of the HUMAC program allowing the Orthotron and KT systems to provide isokinetic testing at a reduced cost. Cybex brand’s international presence expanded and developed in the late 1980s. With sales agents in more than 40 countries. That year Lumex expanded its product offerings to include dedicated Cybex Back Systems and Cybex Fitness Systems

1988 Cybex Medical, commissioned CSMI to design and manufacture the Cybex 1000, 1100, and 1200, a low-cost line of isokinetic testing devices based on the Cybex Orthotron Actuator. CSMI re-designed the actuator incorporating a strain gauge torque sensor and optical encoder position sensor. This gave the dynamometer the ability to detect torque and position all in one unit which meant the strain gauge (which had been external) was now an internal component. This technological advancement lead to force being described as torque (Nm or FtLbs) rather than the previous standard of weight (Kg or Lbs)
1989 the Kin-Com AP is released a single chair and bed isokinetics machine with a movable dynomometer. The design of a movable dynomometer was to become the gold standard in isokinetics in later years.
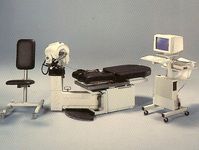
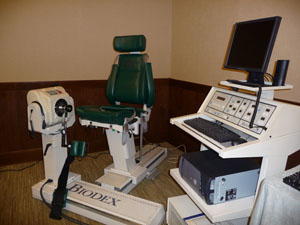
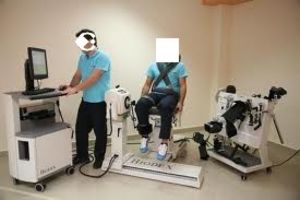
1990 Biodex (Shirley NY) releases the Biodex 2000 a two chair version of their isokinetics system with specifically designed tools to limit range of motion this added a new level of safety to the now more common eccentric mode.
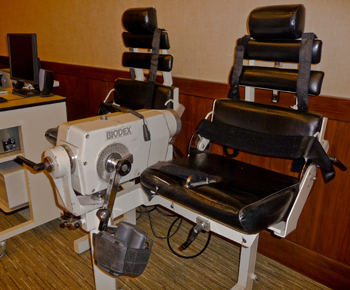
Lumex initiated advisory boards of medical and fitness professionals to help improve existing equipment designs as well as originate new designs. New products included the fourth generation of the Cybex Extremity Testing and Rehabilitation System (Cybex 6000).
1991 CSMI designs the Smart Analog Module (SAM) available to all isokinetic extremity system manufacturers.

1992 The Cybex 6000 was a technological step forwards for Cybex as the system provided both powered (eccentric) and non-powered (concentric) options and continuous passive motion (CPM).
1992 also saw the release of work simulation tools by various manufacturers these machines were often large and offered limited testing capabilities. Later these stand alone units (including the trunk units) would become integrated into true multi joint systems requiring only one dynamometer to power the entire system.



1994 the Kin-Com AP single chair touch screen system is released. This system would remain the ‘gold standard’ system for many years to come. It’s innovative touch screen technology and automatic positioning of a single chair which could be set flat to create a bed/table would signal the future of isokinetic design. To date the design fundamentally remains as the best overall footprint for an isokinetics system. The touch screen allowed faster setup of subjects and automated mixed protocols with good feedback. The system allowed isokinetic, isotonic, isometric and cpm modes with a powered cpm mode for rehabilitation of stiff joints. This machine is the origional design we now all know and love. It remains the industry standard. Later Biodex were to buy the Kin-Com brand to make use of it’s many innovations.

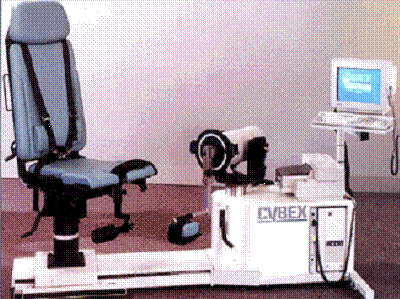
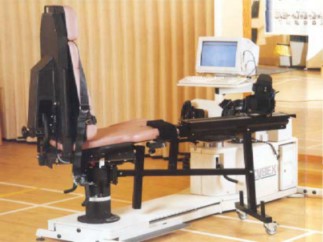
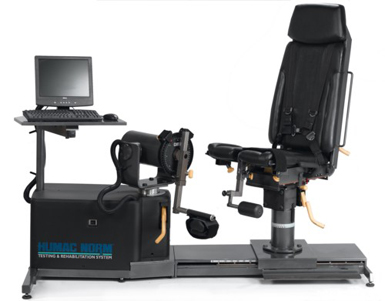
In 1995 Lumex released the Cybex NORM a single chair / dynamometer configuration, after extensive study of the industry design of T-base and elbow-based machines with tethered computer carts. The engineers and product specialists developed a superior and patented sliding chair / fixed dynamometer configuration with integrated computer system.
With the advent of the new multi joint single chair systems the main manufacturers decided to rationalize the attachments to the main unit. Biodex and Kin-Com both decided to make a foot plate for the leg press movement whilst Cybex made a plug in unit.
Biodex incorporated their trunk machines into a seated trunk flexion/extension module. Whilst Cybex produced a plug in trunk module which remains the industry standard to this day (it was later replicated by Con-Trex).

Choosing to focus business operations in the fitness industry, Cybex sold its line of isokinetics testing and physical rehabilitation equipment in 1997. Henley Healthcare purchased the product line and licensed the Cybex brand for the equipment.
1997 CSMI buys the rights to the Cybex II and Cybex II+ systems offering full software and PC updates. 1998 CSMI releases new PC and software systems for the Cybex 300 and Back Systems. This means all previous versions of the Cybex isokinetics systems can now be fully upgraded to modern computers and software. This ‘never obsolete’ approach sets Cybex apart from the other equipment manufacturers and continues to this day.
1999 Biodex releases it’s single chair version of a multi joint system with the system 3. This would feature all of the modes that were associated with the main manufacturers. Biodex also added two proprioceptive modes (joint position sense active and passive) and later introduced the first 3D muscle performance mapping when they licensed for use the Isomap system which showed torque deficits as colors across a speed spectrum.
1999 CSMI then acquired the rights to the complete Cybex isokinetic testing product line (Cybex II, 300, Back Systems, 6000, and NORM) from 2000 CSMI releases the HUMAC upgrade for the Cybex 6000.
2001 CSMI releases the HUMAC upgrade for the Cybex NORM. The HUMAC replaces the original NORM Computer, Windows 3.1 Software, ATIC and NDCB with the HUMAC Windows software and high-speed, dual-processor, USB-based HUMAC Controller increasing data rates from 100hz to 2khz.
2002 CSMI purchases the Cybex NORM Systems product line from Znetics. The purchase includes all designs, patents, inventory, and tooling for the continued production and support of the Cybex NORM system.
2002 the Con-Trex MJ (multi joint) sytem is released by CMV AG (Zürichstrasse) utilising a A/C dynamometer the MJ is only limited in torque by the amount of electricity that can be supplied. This has made the system extremely popular in the research setting offering extremely high torque levels especially in the eccentric mode.

Con-Trex go on to release several variations of there isokinetic unit starting with a new dedicated leg press unit which remains by far the most robust of any produced.
Later releasing a column based system for functional capacity evaluation.

2004 Baltimore Therapeutic Equipment (a company with a 25 year history of established medical devices) and Hanoun Medical merge to create BTE inc. The Primus (RS and Pro available) link a column based system to give functional capacity evaluation.

2007 CSMI releases the new Norm with a changed and developed software interface. Increased reporting capabilities. Proprioceptive modes (joint position sense and kinesthetic sense active and passive). Stand alone modules for researchers and universities. A group screening module to continue to support the sports teams (including the NFL) using the systems world wide. The dynomometer is changed to a brush-less motor allowing the torque level to increase at the higher speed range. A new digital servo amplifier is released increasing data samples from 2000 to 5000 samples per second available for the system and for external acquirement through a dedicated board.
2009 CSMI releases integration of touch screen technology and full EMG support.
2009 Biodex release the new system 4 and system 4 quick set drawing on their many years of experience in the market Biodex release the two models the first aimed at the high end isokinetic market and the second offering limited positions but for a reduced price making isokinetics machine more readily available.
2012 CSMI announces the new Norm2 at Medica in Dusseldorf Germany. The new system is incorporated into a smaller footprint and has been developed to work with the CSMI functional tool set. Set for release in 2013 this will include the new cable reel technology set.

2013 CSMI releases the Norm2 complete with cable reel technology. This allows for 3 dimensional testing functionally whilst also providing a stable platform for ordinary isolated joint testing.

The shaft is able to turn 360 degrees allowing for pulling, pushing and squatting motions as well as all of the functional tools normally only seen in the Occupational Health settings.